Pramoda Devi Wadiyar to sponsor vaccines for 1,000 persons; Technical glitches disappoint citizens
Mysore/Mysuru: The Third Phase of COVID-19 vaccination began across India this morning, covering people over 60 and above. Even those above the age of 45 years who have co-morbidities are eligible to receive the Coronavirus vaccines. In Mysuru, there was a good response from senior citizens.
However, a majority of 60 plus citizens returned disappointed at two Government Hospitals — Mysore Medical College & Research Institute (MMC&RI) Trauma Centre and District Hospital on KRS Road — following technical glitches in CoWIN2.0 portal which did not support registration of names. So, hardly four persons received the jab as against the gathered 30-40 beneficiaries.
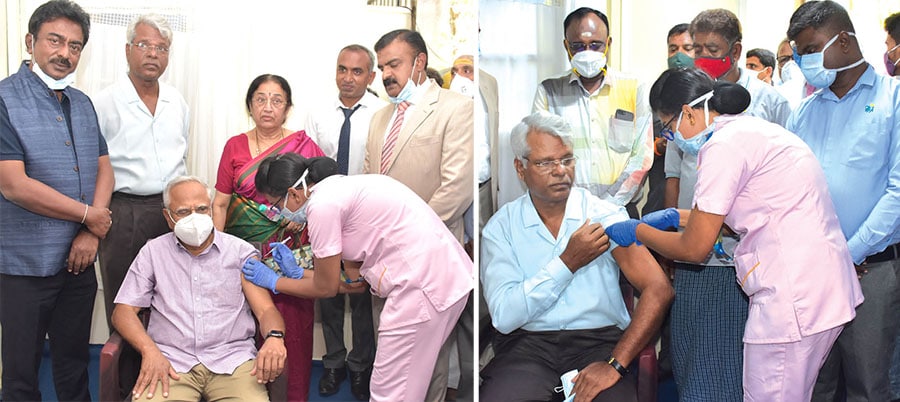
While R. Guru, Chairman, NR Foundation, inaugurated the drive and received the first jab at Apollo BGS Hospital, Dr. Vasundhara Doraswamy, noted danseuse, received first shot at JSS Multi-Speciality Hospital. J. Ramakrishna, a resident of Hebbal, was the first recipient at the District Hospital.
Pramoda Devi Wadiyar, member of the erstwhile Mysore Royal Family announced that she will bear the expenses of vaccine cost of 1,000 persons at JSS Hospital. She wanted all senior citizens to take the vaccine.

Sri Chidananda Swamiji of Hosmutt and Dr. Aryamba Pattabhi, a noted Kannada littérateur, received the vaccine second and third respectively at JSS Hospital. As many as 30 persons registered their names to take vaccine today.
Beneficiaries were declared ‘fit for vaccination’ only after initial medical check-up. Dr. M. Guruswamy, Medical Superintendent, Dr. Col. M. Dayanand, Hospital Director, Shivakumaraswamy, Secretary of JSS Mahavidyapeeta and others were present.
At Apollo Hospital, the vaccine was administered free of cost to all those who have registered to receive vaccine this morning. Noted among who received the jab included, Jagadish Babu, Chairman, Sankalp Group, Sonam Tsering, Tibetan Monk, Dr. Aravinda Malagathi, litterateur, Dr. Vijayalakshmi, retired Dean, Head, Department of OBG, K.R. Hospital, Dr. M. Surendra, retired professor, Department of Botany, UoM, K.T. Srinivas of National Institute of Personal Management and Bhaskar Kalale, past chairman, Confederation of Indian Industries (CII).
4.5 lakh beneficiaries
Meanwhile, Deputy Commissioner Rohini Sindhuri told media persons that as many as 4.5 lakh people are in this category and all will be administered the vaccine in the coming days. Due to technical issues, registration was done both offline and on site.
Those who have taken the first jab must take second dose after 28 days. Since the number of beneficiaries was more in this category, vaccination will be done in more number of hospitals in the coming days. For today, only 30-40 persons will be vaccinated symbolically due to technical glitches and the number will be increased in order to cover all beneficiaries. “We are optimistic that more and more people will come forward to take the vaccine,” she said.
At MMC&RI Trauma Centre, four senior citizens received the vaccine. Dr. C.P. Nanjaraj, Dean and Director, MMC&RI, Dr. Rajesh, Registered Medical Officer, K.R. Hospital, Dr. Shantha, Senior Nursing Officer and others were present.
At District Hospital, Deputy Commissioner Rohini Sindhuri, District Health Officer, Dr. T. Amarnath, Dr. L. Ravi, Reproductive and Child Health Officer and COVID-19 Vaccination Nodal officers and others were present.

How does Covishield work?
The Oxford-AstraZeneca vaccine is being manufactured locally by the Serum Institute of India. The vaccine, which is known as Covishield, is made from a weakened version of a common cold virus (known as an adenovirus) from chimpanzees. It has been modified to look more like coronavirus – although it can’t cause illness.
When the vaccine is injected into a patient, it prompts the immune system to start making antibodies and primes it to attack any coronavirus infection. The jab is administered in two doses given between four and 12 weeks apart.
How does Bharat Biotech’s COVAXIN work?
The govt-backed COVAXIN vaccine, developed by Bharat Biotech is an inactivated vaccine made up of killed Coronaviruses, making it safe to be injected into the body. Bharat Biotech used a sample of the coronavirus, isolated by India’s National Institute of Virology. When administered, immune cells can still recognise the dead virus, prompting the immune system to make antibodies against the pandemic virus.
These people will get priority in vaccination provided they carry doctor’s certificate
- Heart Failure with hospital admission in past one year.
- Post Cardiac Transplant / Left Ventricular Assist Device (LVAD).
- Significant Left ventricular systolic dysfunction (LVEF <40%).
- Moderate or Severe Valvular Heart Disease.
- Congenital heart disease with severe PAH or Idiopathic PAH.
- Coronary Artery Disease with past CABG/PTCA/MI and Hypertension/Diabetes on treatment.
- Angina and Hypertension/Diabetes on treatment.
- CT/MRI documented stroke and Hypertension/Diabetes on treatment.
- Pulmonary artery hypertension and Hypertension/Diabetes on treatment.
- Diabetes (> 10 years or with complications) and Hypertension on treatment.
- Kidney/ Liver/ Hematopoietic stem cell transplant: Recipient/on wait-list.
- End Stage Kidney Disease on haemodialysis/ CAPD.
- Current prolonged use of oral corticosteroids/immunosuppressant medications.
- Decompensated cirrhosis.
- Severe respiratory disease with hospitalisations in last two years/FEV1 <50%.
- Lymphoma/Leukaemia/Myeloma.
- Diagnosis of any solid cancer on or after 1st July 2020 or currently on any cancer therapy.
- Sickle Cell Disease/Bone marrow failure/Aplastic Anemia/Thalassemia Major.
- Primary Immunodeficiency Diseases/HIV infection.
Persons with disabilities due to intellectual disabilities/ Muscular Dystrophy/Acid attack with involvement of respiratory system/ Persons with disabilities having high support needs/ Multiple disabilities including deaf-blindness.








Recent Comments